Description
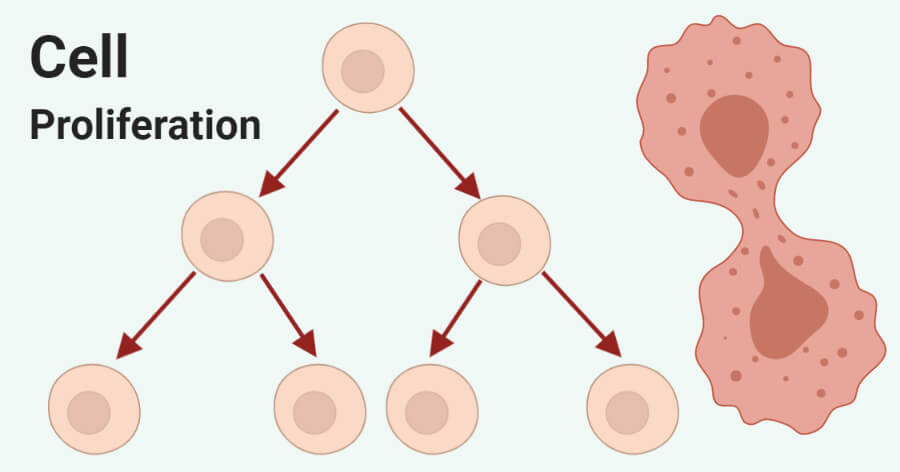
Cell proliferation Recombinants is a physiological process of cell division that occurs in almost all tissues, resulting in an increase in cell number. Mitosis is the main way that eukaryotes divide cells, such as multicellular organisms, mitotically to increase the number of somatic cells. During mitotic cell division, a single parent cell divides to produce two daughter cells to replace cells that have been damaged or died, thus cell proliferation is defined by the balance between cell divisions and cell loss through cell death or differentiation. The process of cell proliferation plays a key role from the time of embryogenesis to the development of the whole organism from the one- or two-cell embryo and continues its critical role in maintaining adult tissue homeostasis by recycling old cells with new cells.
Overview of cell proliferation
Early development is characterized by the rapid proliferation of embryonic cells, which then differentiate to produce many specialized cell types that make up the tissues and organs of multicellular animals. As cells differentiate, their rate of proliferation usually decreases, and most cells in adult animals are arrested at the G0 stage of the cell cycle. Some differentiated cell types never divide again, but most cells can resume proliferation as needed to replace cells that have been lost as a result of injury or cell death. Also, some cells divide continuously throughout life to replace cells that have a high turnover rate in adult animals. Cell proliferation is carefully balanced with cell death to maintain a constant number of cells in adult tissues and organs.
In general, adult animal cells can be grouped into three categories with respect to cell proliferation.
- The first category, like human cardiomyocytes, can no longer proliferate. They are produced, differentiated and retained throughout the life of the organism during embryonic development. They will never be replaced, even if they have been lost due to injury.
- In contrast to the first category, the second can resume proliferation when it enters the G0 stage of the cell cycle, including skin fibroblasts, smooth muscle cells, endothelial cells lining blood vessels, and epithelial cells of the skin. most internal organs, such as the liver, pancreas, kidney, lung, prostate, and breast.
- The third category, which includes blood cells, epithelial cells of the skin, and epithelial cells lining the digestive tract, has a short lifespan and must be replaced by continuous cell proliferation in adult animals. Within them, fully differentiated cells do not proliferate on their own. Instead, they are replaced through the proliferation of less differentiated cells, called stem cells.
Regulation of Cell Proliferation
- Growth factors
A wide range of growth factors combines with receptor tyrosine kinases (RTKs), which are the second major type of cell surface receptors, to regulate cell proliferation, differentiation, and survival. Fibroblast growth factors (FGFs) are a family of growth factors involved in wound healing and angiogenesis. Among the various members of this family, FGF-2 and FGF-4 have been shown to increase the proliferation of human cells in vitro. FGF signals proliferation through the mitogen-activated protein kinase (MAPK) cascade in various cell types.
FGFs bind to fibroblast growth factor receptors (FGFRs), epidermal growth factors (EGFs) bind to epidermal growth factor receptors (EGFRs), vascular endothelial growth factor (VEGF), and vascular endothelial growth factor (VEGF). platelet-derived growth factor (PDGF) binds to PDGFR and hepatocyte growth factor (HGF) binds to c-Met, causing phosphoinositide-3 kinase (PI3K) to be activated, converting PIP2 to PIP3 and activating Akt/protein kinase B (PKB) and mitogen-activated protein kinase (MAPK) Erk. Phosphorylated Erk enters the nucleus and activates transcription of cell proliferation genes such as c-myc, or activates downstream receptors such as Risk which then activate proliferation genes.
- Bone morphogenetic protein (BMP)
Bone morphogenetic protein (BMP) belonging to the TGFβ superfamily is known to affect bone formation. Both BMP-2 and BMP-3 can increase cell proliferation. Since all of these factors affect bone formation at different rates and some have a greater effect on proliferation, synergistic pairs of these growth factors can be used at optimal doses and at specific points during the bone regeneration process.
Serine also plays an essential role in the synthesis of biomolecules that promote cell proliferation. TGFβ signalling occurs when TGFβ or family factors bind to a type II serine-threonine kinase receptor that recruits another transmembrane protein (receptor I). Receptor I phosphorylates primary intracellular downstream SMAD molecules, causing their translocation into the nucleus and transcription of specific genes. Receptor I can be ALK-1, ALK-2, ALK-3, or ALK-6 that signals SMAD 1, SMAD 5, and SMAD 8, or it can be ALK-4, ALK-5, or ALK-7 that signals SMAD 2 and SMAD 3.
Signalling through SMAD 1, SMAD 5, or SMAD 8 is required for chondrocyte differentiation, whereas signalling through SMAD 2 or SMAD 3 blocks chondrocyte differentiation. TGFβ and members of this family of growth factors can also signal through mitogen-activated protein kinase (MAPK), Rho GTPase, and phosphoinositide-3 kinase (PI3K) pathways. Furthermore, the mitogenic effects of BMP-3 have been found to be mediated by TGFβ/activin signalling and not by any of the MAPK signalling pathways, with ALK-4 and SMAD 2 and SMAD 3 being the key players involved.
- Notch1 intracellular domain (NICD)
Notch signalling is activated primarily when DSL family ligands (Delta, Serrate/Jagged, LAG-2) activate Notch receptors on adjacent cells. This causes the release and nuclear localization of the Notch intracellular domain (NICD), which together with transcriptional regulators modifies the expression of target genes. Overexpression of the Notch1 intracellular domain (NICD) has been shown to inhibit cell proliferation and alter the neuroendocrine phenotype of medullary thyroid cancer cells.
Cell proliferation in the pathophysiology
Cancer can result from the abnormal proliferation of any type of cell. The most important distinction for the patient is between benign tumours, which remain confined to their site of origin, and malignant tumours, which can invade normal tissues and spread throughout the body. PSAT1 is the protein-encoding gene for phosphoserine aminotransferase, which catalyzes serine biosynthesis. Previous studies have shown that PSAT1 plays a vital role in cell proliferation, acting as an oncogene in colon cancer and non-small cell lung cancer (NSCLC).
PSAT1 is overexpressed in colon cancers where it contributes to cell proliferation and chemoresistance, resulting in poor prognosis. PSAT1 is significantly upregulated in ER-negative breast cancer and correlated with poor patient prognosis. Furthermore, PSAT1 was found to be regulated by ATF4, which then activated the GSK-3β/β-catenin pathway. This resulted in the enhancement of cyclin D1 expression and the promotion of cell proliferation. Statistical analysis of many results showed that PSAT1 upregulation was correlated with tumour development and poor prognosis. These observations have led us to speculate on the role of PSAT1 in the initiation and development of ER-negative breast cancer.